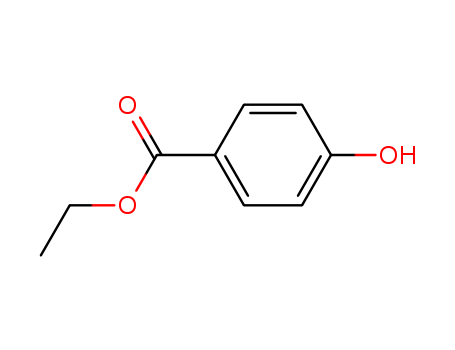

CasNo: 120-47-8
MF: C9H10O3
Appearance: white crystalline powder
|
Production Methods |
Ethylparaben is prepared by the esterification of p-hydroxybenzoic acid with ethanol (95%). |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 39, p. 3343, 1974 DOI: 10.1021/jo00937a007 |
|
Pharmaceutical Applications |
Ethylparaben is widely used as an antimicrobial preservative in cosmetics,food products, and pharmaceutical formulations. It may be used either alone or in combination with other paraben esters or with other antimicrobial agents. In cosmetics it is one of the most frequently used preservatives. The parabens are effective over a wide pH range and have a broad spectrum of antimicrobial activity, although they are most effective against yeasts and molds. Owing to the poor solubility of the parabens, paraben salts, particularly the sodium salt, are frequently used. However, this may cause the pH of poorly buffered formulations to become more alkaline. |
|
Safety |
Ethylparaben and other parabens are widely used as antimicrobial preservatives in cosmetics, food products, and oral and topical pharmaceutical formulations. Systemically, no adverse reactions to parabens have been reported, although they have been associated with hypersensitivity reactions. Parabens, in vivo, have also been reported to exhibit estrogenic responses in fish.(10) The WHO has set an estimated total acceptable daily intake for methyl-, ethyl-, and propylparabens at up to 10 mg/kg body-weight.LD50 (mouse, IP): 0.52 g/kg LD50 (mouse, oral): 3.0 g/kg |
|
storage |
Aqueous ethylparaben solutions at pH 3–6 can be sterilized by autoclaving, without decomposition. At pH 3–6, aqueous solutions are stable (less than 10% decomposition) for up to about 4 years at room temperature, while solutions at pH 8 or above are subject to rapid hydrolysis (10% or more after about 60 days at room temperature). Ethylparaben should be stored in a well-closed container in a cool, dry place. |
|
Incompatibilities |
The antimicrobial properties of ethylparaben are considerably reduced in the presence of nonionic surfactants as a result of micellization. Absorption of ethylparaben by plastics has not been reported, although it appears probable given the behavior of other parabens. Ethylparaben is coabsorbed on silica in the presence of ethoxylated phenols. Yellow iron oxide, ultramarine blue, and aluminum silicate extensively absorb ethylparaben in simple aqueous systems, thus reducing preservative efficacy. Ethylparaben is discolored in the presence of iron and is subject to hydrolysis by weak alkalis and strong acids. |
|
Regulatory Status |
Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral, otic, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Definition |
ChEBI: Ethylparaben is an ethyl ester resulting from the formal condensation of the carboxy group of 4-hydroxybenzoic acid with ethanol, It has a role as an antimicrobial food preservative, an antifungal agent, a plant metabolite and a phytoestrogen. It is a paraben and an ethyl ester. |
InChI:InChI:1S/C9H10O3/c1-2-12-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
A facile method for the synthesis of hig...
-
Experimental and theoretical quantum che...
The parabens, alkylic esters of p-hydrox...
The synthesis of novel 1,2,3-triazole-hy...
Structural based molecular docking appro...
Abstract: A simple and green approach to...
-
N-Methoxycarbonyl-5-ethoxycarbonyl-2,3-d...
Benzoic acid or p-hydroxybenzoic acid un...
(Chemical Equation Presented) Several Br...
A new series of antioxidants, namely imi...
A new series of mesogenic compounds havi...
Alkaloids from the marine sponge Zyzzya ...
-
Solvothermal reaction of zinc(ii) and ca...
This paper describes the synthesis and c...
-
The invention belongs to the field of me...
In this paper, some new bis-acyl hydrazo...
Raloxifene agonism of estrogen receptor ...
A novel series of arylcarbamate-N-acylhy...
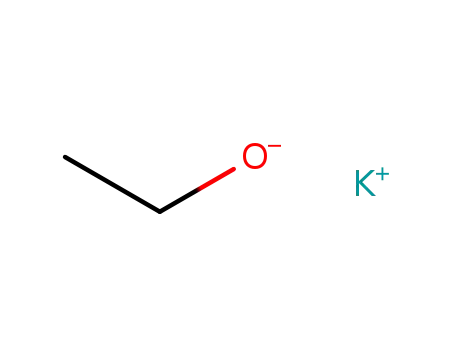
potassium ethoxide

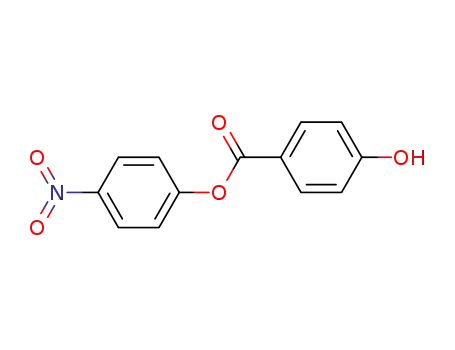
4-nitrophenyl 4-hydroxybenzoate

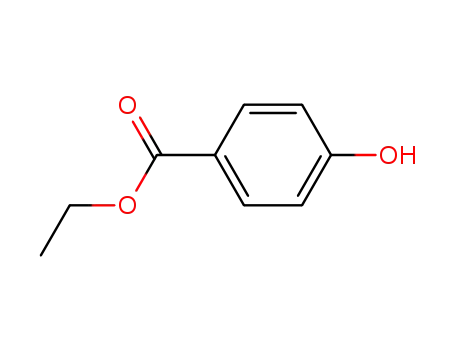
Ethyl 4-hydroxybenzoate

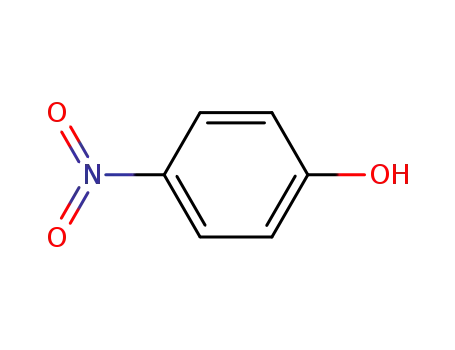
4-nitro-phenol
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 25 ℃;
Reagent/catalyst;
Kinetics;
|
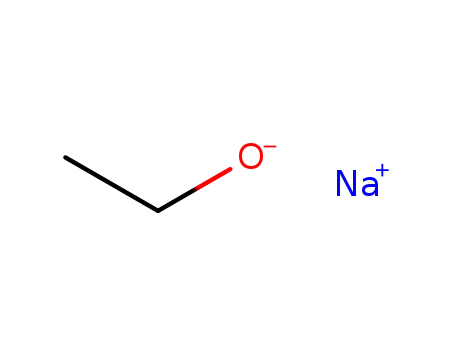
sodium ethanolate


4-nitrophenyl 4-hydroxybenzoate


Ethyl 4-hydroxybenzoate


4-nitro-phenol
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 25 ℃;
Kinetics;
|
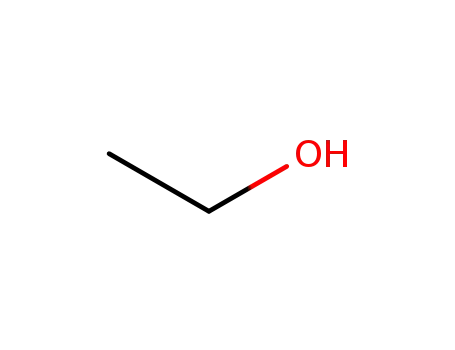
ethanol
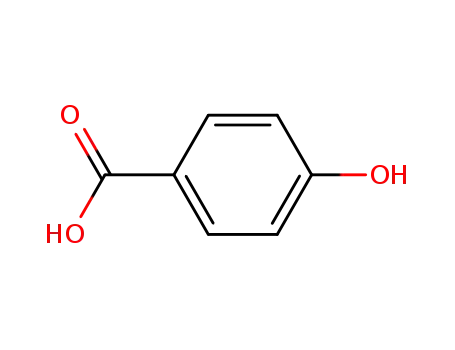
4-hydroxy-benzoic acid
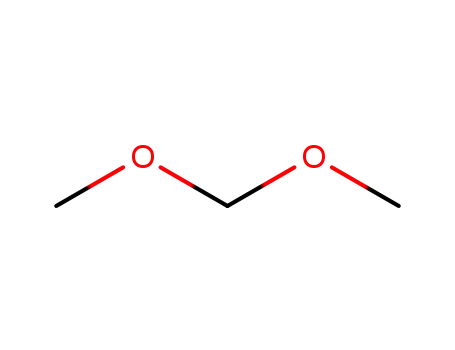
Dimethoxymethane
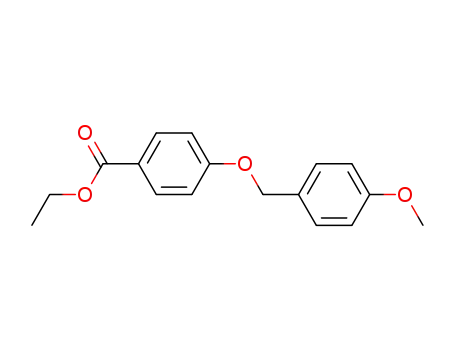
ethyl 4-((4-methoxybenzyl)oxy)-benzoate
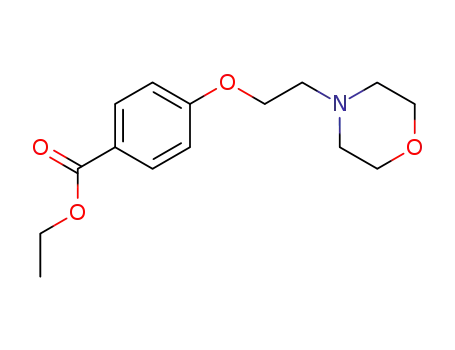
4-(2-morpholin-4-yl-ethoxy)-benzoic acid ethyl ester
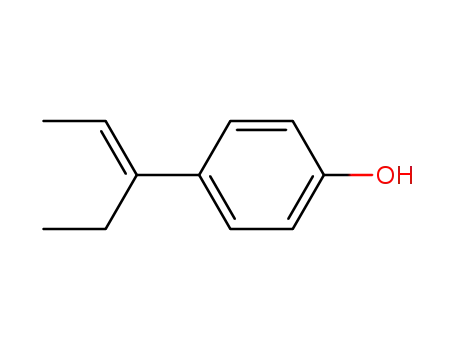
E-β-Methyl-α-ethyl-4-hydroxystyrene
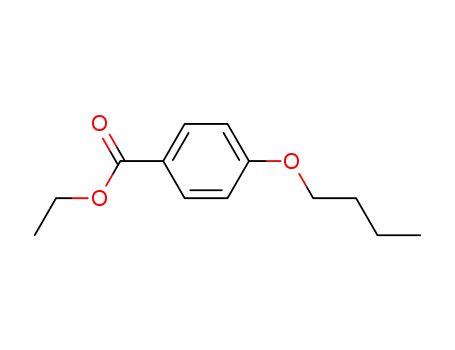
ethyl 4-butoxybenzoate
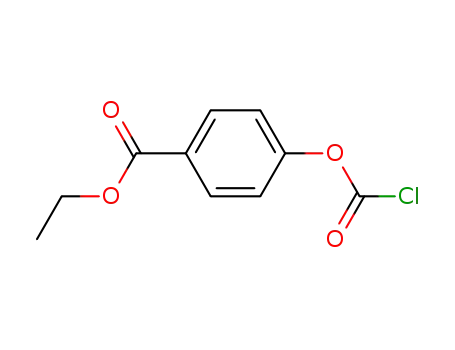
4-(ethoxycarbonyl)phenyl chloroformate