Home > Products > Human APIs
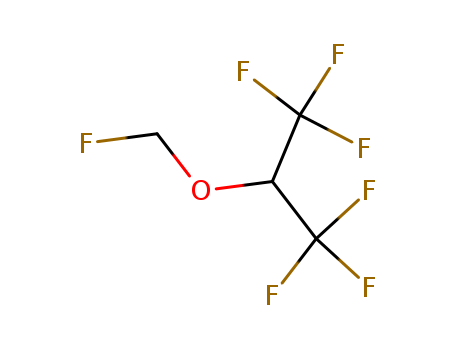

CasNo: 28523-86-6
MF: C4H3F7O
Appearance: colorless liquid
|
Manufacturing Process |
164 g (2.31 mole) of chlorine is slowly bubbled into a flask containing 370 g (2.03 mole) of methyl 1,1,1,3,3,3-hexafluoroisopropyl ether illuminated with a 250 watt incandescent lamp, starting at room temperature. The product is washed with a potassium carbonate solution until neutral, dried over MgSO4 and vacuum distilled to yield 304 g (1.5 mole) of chloromethyl 1,1,1,3,3,3- hexafluoroisopropyl ether (chloromethyl 1,1,1,3,3,3-hexafluoro-2-propyl ether), boiling point 78°C.A solution of chloromethyl 1,1,1,3,3,3-hexafluoro-2-propyl ether (754 g, 3.49 moles) in dry tetrahydrothiophene 1,1-dioxide (203 g, 3,49 moles) were stirred and heated to 130°C in a creased flask fitted with a fractional distillation assembly. A distillate (200 ml), b748 56.0° to 62°C, was collected during 5 h. Then the reaction mixture was cooled to room temperature, dry potassium fluoride (100 g, 1.74 moles) was added, and the cycle of operations was repeated 3 times at temperatures between 138° to 185°C to give distillates (100 ml, 100 ml and 50 ml), b746 58° to 61°C, 55.5° to 57°C, and 54.2° to 55.9°C, respectively. From this portionwise addition of potassium fluoride (503 g, 8.7 moles) there was obtained distillates totalling 672 g, b746 54.2° to 62.0°C, which by GLC analysis was about 92% fluoromethyl and 6.8% chloromethyl 1,1,1,3,3,3-hexafluoro-2-propyl ether.Fractional distillation of 659 g gave a forerun (46 g), b745 53.5° to 57.0°C, and then 99.6% pure fluoromethyl 1,1,1,3,3,3-hexafluoro-2-propyl ether (505 g), b745 57.0° to 57.7°C. |
|
Therapeutic Function |
Anesthetic |
|
Biological Functions |
Sevoflurane (Ultane) is the most recently introduced inhalation anesthetic. It has low tissue and blood solubility, which allows for rapid induction and emergence and makes it useful for outpatient and ambulatory procedures. It has the advantage of not being pungent, a characteristic that permits a smooth inhalation induction, and is particularly useful in pediatric anesthesia. Hypotension is produced by sevoflurane as systemic vasodilation occurs and cardiac output decreases. Since it does not directly produce tachycardia, it is a useful alternative to consider in patients with myocardial ischemia. However, a concern for reflex-induced tachycardia remains. Sevoflurane undergoes hepatic biotransformation (about 3% of the inhaled dose), and it is somewhat degraded by conventional CO2 absorbents. The degradation product from the absorbent has been reported to be nephrotoxic, although the report is controversial and not substantiated by more recent studies. Sevoflurane’s actions on skeletal muscle and on vascular regulation within the CNS are similar to those described for the other halogenated hydrocarbon anesthetics. |
|
Veterinary Drugs and Treatments |
Sevoflurane may be useful in a variety of species when rapid induction and/or rapid recoveries are desired with an inhalational anesthetic. |
|
Definition |
ChEBI: An ether compound having fluoromethyl and 1,1,1,3,3,3-hexafluoroisopropyl as the two alkyl groups. |
|
Brand name |
Ultane (Abbott);Sevofrane. |
|
General Description |
Sevoflurane is a volatile, nonpungent, nonflammable, andnonexplosive liquid with a boiling point of 58.6°C. Theblood:gas partition coefficient is 0.65, the oil:gas partitioncoefficient is 50, and the MAC is 2.1%. Sevoflurane reactswith desiccated carbon dioxide adsorbents, to produce compounds(A and B) with known toxicity . The typeof CO2 absorbent used, the temperature of the absorbent,and the duration of exposure can influence the degree towhich sevoflurane breaks down. The major breakdownproduct, compound A, pentafluoroisopropenyl fluoromethylether, (PIFE, C4H2F6O) has been studied extensively.Compound A is nephrotoxic in rats and nonhuman primatesand remains a theoretical risk to humans. As discussedpreviously under the stability of inhaled anesthetics,sevoflurane breakdown by CO2 absorbents generates heatand has resulted in sporadic operating room fires. |
InChI:InChI=1/C4H3F7O/c5-1-12-2(3(6,7)8)4(9,10)11/h2H,1H2
The invention discloses a novel green an...
The invention relates to a preparation m...
A process for removing water from a mixt...
The present invention provides a method ...
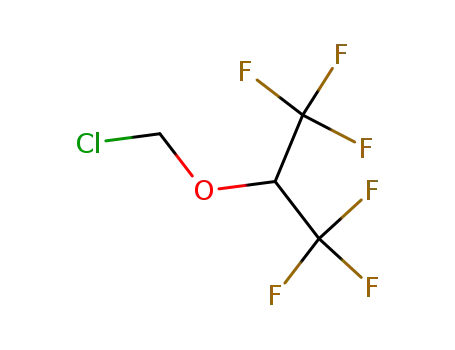
1,1,1,3,3,3-hexafluoroisopropyl chloromethyl ether

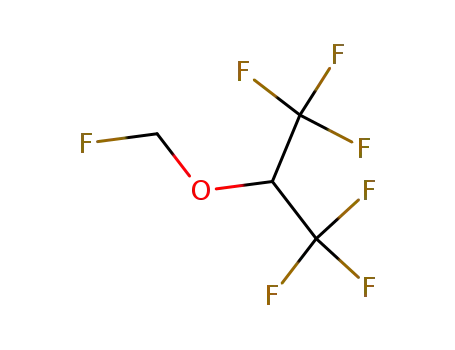
sevoflurane
| Conditions | Yield |
|---|---|
|
With
diisopropylethylamine hydrofluoride;
for 16h;
Heating;
|
95% |
|
With
potassium fluoride; potassium hydrogenfluoride; water;
Aliquat HTA-1;
at 100 ℃;
for 3h;
Product distribution / selectivity;
|
90% |
|
With
potassium fluoride;
tetraethylammonium chloride;
In
water;
at 100 ℃;
for 3h;
Product distribution / selectivity;
|
86% |
|
With
potassium fluoride;
Aliquat HTA-1;
In
water;
at 60 - 100 ℃;
for 1 - 17h;
Product distribution / selectivity;
|
85% |
|
With
potassium fluoride;
tetrabutylammomium bromide;
In
water;
at 60 - 100 ℃;
for 3 - 15h;
Product distribution / selectivity;
|
83% |
|
With
potassium fluoride;
tricaprylmethylammoniumchloride;
In
water;
at 60 - 100 ℃;
for 3 - 16h;
Product distribution / selectivity;
|
76% |
|
With
potassium fluoride; potassium iodide;
In
PEG 400 (polyethylene glycol having an average molecular weight of 400);
for 1.5 - 2h;
Product distribution / selectivity;
Heating / reflux;
|
66% |
|
With
potassium fluoride; potassium iodide;
In
MDGCM (a mono- and diglyceride of medium chain length);
for 1.5 - 3h;
Product distribution / selectivity;
Heating / reflux;
|
60% |
|
With
potassium fluoride; potassium iodide;
In
propylene glycol, propyl gallate;
for 2h;
Product distribution / selectivity;
Heating / reflux;
|
52% |
|
With
potassium fluoride;
In
acetamide; 2,2'-[1,2-ethanediylbis(oxy)]bisethanol;
at 85 ℃;
for 6h;
|
|
|
With
potassium fluoride;
In
acetamide; 2,2'-[1,2-ethanediylbis(oxy)]bisethanol;
at 85 ℃;
for 6h;
Product distribution / selectivity;
|
|
|
With
hydrogen fluoride; N-ethyl-N,N-diisopropylamine;
for 20h;
Concentration;
Pressure;
Temperature;
Time;
Reagent/catalyst;
Reflux;
|
|
|
With
potassium fluoride;
Heating;
Large scale;
|
8.29 kg |
|
With
potassium fluoride; potassium iodide;
In
sulfolane;
for 4h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
propylene glycol, propyl gallate;
for 3h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
LB (a mixture of mono-, di- and triglycerides and mono- and diesters of polyethylene glycol);
for 6h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
MP (1-methoxy-2-propanol);
for 4h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
PEG 400 (polyethylene glycol having an average molecular weight of 400);
for 2h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
PPG 400 (polypropylene glycol having an average molecular weight of 400);
for 4h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
DMI (1,3-dimethyl-imidazolidin-2-one);
for 5h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
N,N-dimethyl-formamide;
for 3h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
sulfolane;
for 6h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
propylene glycol, propyl gallate;
for 24h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
LB (a mixture of mono-, di- and triglycerides and mono- and diesters of polyethylene glycol);
for 20h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
MDGCM (a mono- and diglyceride of medium chain length);
for 24h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
MP (1-methoxy-2-propanol);
for 46h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
PEG 400 (polyethylene glycol having an average molecular weight of 400);
for 3h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
PPG 400 (polypropylene glycol having an average molecular weight of 400);
for 46h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
DMI (1,3-dimethyl-imidazolidin-2-one);
for 9h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride;
In
N,N-dimethyl-formamide;
for 10h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; potassium iodide;
In
MDGCM (a mono- and diglyceride of medium chain length);
for 4 - 6h;
Conversion of starting material;
Heating / reflux;
|
|
|
With
potassium fluoride; Pentaerythritol;
In
sulfolane; water;
at 97 - 102 ℃;
for 10h;
Large scale;
|
64 kg |

1,1,1,3,3,3-hexafluoroisopropyl chloromethyl ether

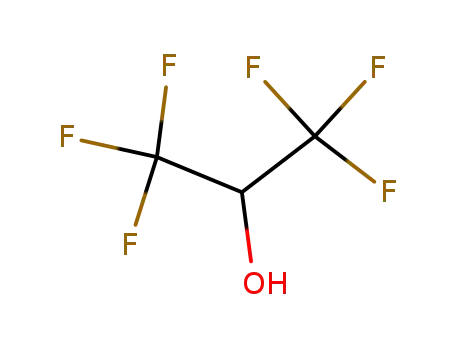
1,1,1,3',3',3'-hexafluoro-propanol


sevoflurane
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; potassium fluoride; water;
Aliquat HTA-1;
at 100 ℃;
for 3h;
Product distribution / selectivity;
|
93% 1.05 %Chromat. |
|
With
potassium fluoride; potassium hydrogenfluoride; water;
Aliquat 175;
at 100 ℃;
for 3h;
Product distribution / selectivity;
|
89% 3.8 %Chromat. |
|
With
potassium fluoride; potassium hydrogenfluoride; water;
benzyl triethyl ammonium dichloride;
at 100 ℃;
for 3h;
Product distribution / selectivity;
|
88% 4 %Chromat. |
|
With
potassium fluoride; water;
tetraethylammonium chloride;
at 100 ℃;
for 3h;
Product distribution / selectivity;
|
86% 0.08 %Chromat. |
|
With
potassium fluoride; water;
tetrabutylammomium bromide;
at 60 ℃;
for 15h;
Product distribution / selectivity;
|
85% 0.4 %Chromat. |
|
With
potassium fluoride; water;
tricaprylmethylammoniumchloride;
at 60 - 120 ℃;
for 3 - 16h;
Product distribution / selectivity;
|
71% 0.12 - 9.9 %Chromat. |
|
With
potassium fluoride; potassium hydrogenfluoride; water;
at 90 ℃;
for 12h;
Product distribution / selectivity;
|
71.7% 4.7 %Chromat. |
|
With
potassium fluoride; water;
Aliquat HTA-1;
at 100 ℃;
for 3 - 6h;
Product distribution / selectivity;
|
57% 0.03 - 11.5 %Chromat. |
|
With
potassium fluoride; potassium hydrogenfluoride; water;
Aliquat HTA-1;
at 90 - 100 ℃;
for 3 - 13h;
Product distribution / selectivity;
|
19.5% 1.6 - 74.4 %Chromat. |
1,3,5-tri-O-benzoyl-2-deoxy-2-fluoro-α-D-arabinofuranose

1,1,1,3,3,3-hexafluoroisopropyl chloromethyl ether

1,1,1,3',3',3'-hexafluoro-propanol
bis(2-chloromethyl)ether
1,1,1,3,3-pentafluoro-2-(fluoromethoxy)-3-methoxypropane
fluoromethyl 1-methoxy-1,3,3,3-tetrafluoro-2-propenyl ether
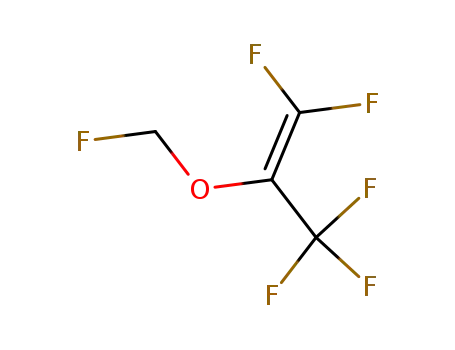
fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether
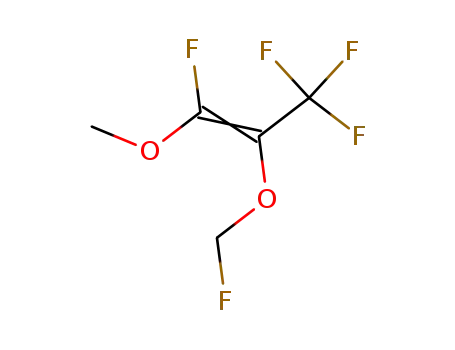
(Z)-1,3,3,3-Tetrafluoro-2-fluoromethoxy-1-methoxy-propene