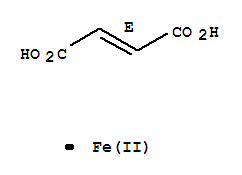

CasNo: 141-01-5
MF: C4H2FeO4
Appearance: orange red to reddish-brown powder with no odour and no taste
|
Manufacturing Process |
Sodium carbonate (53.5 pounds of Na2CO3-H2O) was dissolved in water (40 to 45 gallons) and fumaric acid (50 pounds) was added slowly. During the addition the solution was stirred and heated. The resulting solution of sodium fumarate, having a pH of 6.8, was added slowly with mixing to a solution of ferrous sulfate (118 pounds FeSO4-7H2O in 33 gallons of water) having a pH of 3.3, both solutions being maintained at or near boiling temperature during the mixing. The resulting slurry of reddish-brown anhydrous ferrous fumarate was filtered and washed in a centrifuge and dried in a tray drier (15 hours at 110°C). Yield: 63 pounds, 86% of theory. Calculated for FeC4H2O4: Fe, 32.9%. Found: Fe, 32.6%. Only 0.2% of ferric iron (Fe+++) was found. |
|
Therapeutic Function |
Hematinic |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. Human systemic effects by ingestion: dyspnea, nausea or vomiting, somnolence. When heated to decomposition it emits acrid smoke and irritating fumes. See also FUMRIC ACID. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: reduced absorption of 4-quinolones and tetracyclines. Dimercaprol: avoid concomitant use. Mycophenolate: may significantly reduce absorption of mycophenolate. |
|
Metabolism |
Molecular weight (daltons) 169.9 % Protein binding - % Excreted unchanged in urine - Volume of distribution (L/kg) - Half-life - normal/ESRF (hrs) - |
|
Definition |
Anhydrous salt of a combination of ferrous iron and fumaric acid, stable, odorless, substantially tasteless. Reddish-brown, anhydrous powder, contains 33% iron by weight, does not melt at temperatures up to 280C, insoluble in alcohol, very slightly solubl |
|
General Description |
Ferrous fumarate is a commonly used inexpensive substitute for other forms of iron, that is employed as a food iron fortificant.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
InChI:InChI=1/C4H4O4.Fe/c5-3(6)1-2-4(7)8;/h1-2H,(H,5,6)(H,7,8);/q;+2/p-2