Home > Products > Human APIs
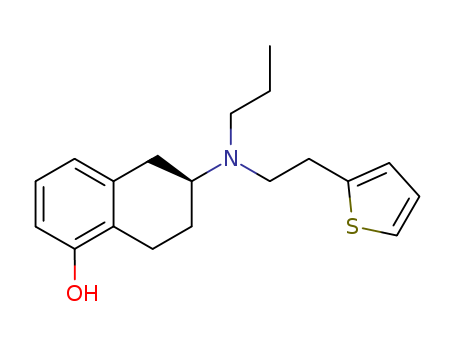

CasNo: 99755-59-6
MF: C19H25NOS
|
Synthesis |
The synthesis described by the originators at Discovery Therapeutics Inc. (now known as Aderis Pharmaceuticals) is shown in the scheme. The synthesis utilizes the chiral methoxy tetralin 62 as starting precursor which was obtained via chiral crystallization procedure described in a patent literature [34]. Demethylation of tetraline 62 with refluxing 40% HBr solution for several hours provided phenol 63 in 96% yield. Reaction of the amine 63 with 2- thiophenylethyl tosylate 64 in refluxing xylene for 24-32 h in the presence of 0.6 equiv sodium carbonate gave the desired rotigotine (IX) without requiring chromatographic purification. The ratio of sodium carbonate to the amine was critical to achieving good yields (59-84% yield) without requiring extensive purification. Rotigotine was isolated as the HCl salt. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antipsychotics: avoid concomitant use (antagonism of effect). Metoclopramide: avoid concomitant use (antagonism of effect). |
|
Metabolism |
Rotigotine is metabolised in the gut wall and liver by N-dealkylation as well as direct and secondary conjugation. Main metabolites are sulfates and glucuronide conjugates of the parent compound as well as N-desalkyl-metabolites, which are biologically inactive. Approximately 71% of the rotigotine dose is excreted in urine and a smaller part of about 23% is excreted in faeces. |
|
Brand name |
Neupro |
|
General Description |
Rotigotine, (6S)-6-{propyl[2-(2-thienyl)ethyl]amino}-5,6,7,8-tetrahydro-1-naphthalenol (Neupro),is a nonergoline that is available as a silicone-based, selfadhesivematrix, transdermal system for continuous delivery over a 24-hour period. Approximately 45% of the drug is releasedwithin 24 hours. The terminal half-life of rotigotine is5 to 7 hours after removal of the patch. Rotigotine is 90%bound to plasma proteins. The compound undergoes extensivemetabolism and has low bioavailability by the oralroute. The major metabolites of rotigotine are the glucuronideand sulfate conjugates of rotigotine and sulfateconjugates of N-despropylrotigotine and N-desthienylethylrotigotine. Rotigotine is excreted in the urine (71%) andfeces (11%).Studies using human liver microsomes didnot find any interactions with CYP1A2, CYP2C9,CYP2C19, CYP2D6, and CYP3A4 substrates.Rotigotinetransdermal system contains sodium metabisulfite, and individualssensitive to sulfite could be at risk for allergic reactions.Additionally, somnolence is a common adverse reactionwith individuals on rotigotine, and patients should beclosely monitored during therapy.In transfected Chinesehamster ovary (CHO) cells, rotigotine binds with high affinityat D3 and D2L receptors (variants in the D2 receptor subtypeare caused by insertion of the 29 amino acids into thethird loop to give D2s and D2L).Using rat CHO cells,rotigotine shows over 30-fold selectivity at D3 versus D2 receptors.48 Rotigotine was approved in May 2007 for thetreatment of early-stage PD. |
InChI:InChI=1/C19H25NOS/c1-2-11-20(12-10-17-6-4-13-22-17)16-8-9-18-15(14-16)5-3-7-19(18)21/h3-7,13,16,21H,2,8-12,14H2,1H3/t16-/m0/s1
The invention relates to the technical f...
2-Aminotetralin and 3-aminochroman deriv...
The invention discloses a preparation me...
The present invention relates to a novel...
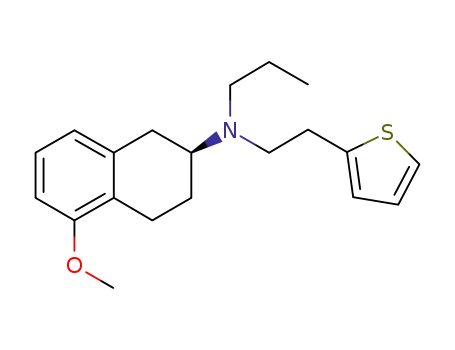
(2S)-5-methoxy-N-propyl-N-(2'-(thien-2-yl)ethyl)tetralin-2-amine

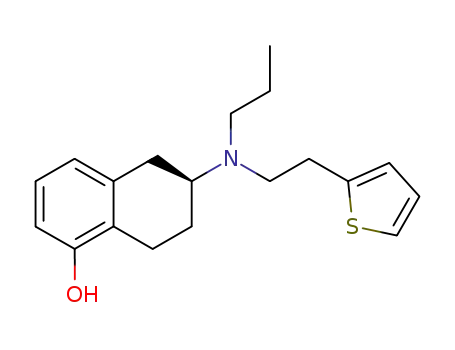
rotigotine
| Conditions | Yield |
|---|---|
|
With
hydrogen bromide;
In
acetic acid;
for 4h;
Reflux;
Green chemistry;
|
93% |
|
With
aluminum (III) chloride;
In
toluene;
at 20 - 45 ℃;
for 8h;
|
66.5% |
|
With
hydrogen bromide;
for 4h;
Concentration;
Reflux;
|
32.72% |
|
(2S)-5-methoxy-N-propyl-N-(2'-(thien-2-yl)ethyl)tetralin-2-amine;
With
aluminum (III) chloride; thiourea;
In
toluene;
at 20 - 105 ℃;
for 3.5h;
With
ammonia;
In
water; toluene;
at 20 - 25 ℃;
pH=8 - 9;
|
|
|
(2S)-5-methoxy-N-propyl-N-(2'-(thien-2-yl)ethyl)tetralin-2-amine;
With
boron tribromide;
In
dichloromethane;
at 0 - 20 ℃;
With
sodium hydrogencarbonate;
In
dichloromethane; water;
|
|
|
With
boron tribromide;
In
dichloromethane;
at 0 - 20 ℃;
|
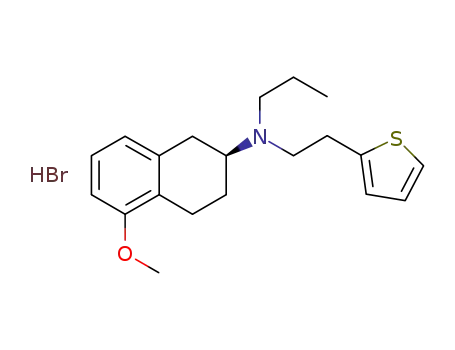
(S)-2-(N-n-propyl-N-2-(thien-2-yl)ethylamino)-5-methoxytetraline hydrobromide


rotigotine
| Conditions | Yield |
|---|---|
|
(S)-2-(N-n-propyl-N-2-(thien-2-yl)ethylamino)-5-methoxytetraline hydrobromide;
With
boron tribromide;
In
dichloromethane;
at 0 - 5 ℃;
With
water;
In
dichloromethane;
With
potassium carbonate;
In
water; ethyl acetate;
pH=7 - 7.5;
|
90% |
|
(S)-2-(N-n-propyl-N-2-(thien-2-yl)ethylamino)-5-methoxytetraline hydrobromide;
With
boron tribromide;
In
dichloromethane;
at 0 - 5 ℃;
for 6h;
With
water;
In
dichloromethane;
With
potassium carbonate;
In
dichloromethane; water;
pH=7 - 7.5;
|
90% |
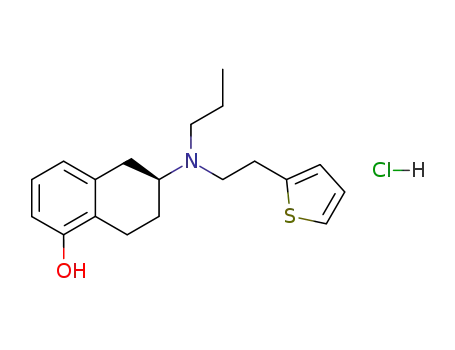
rotigotine hydrochloride
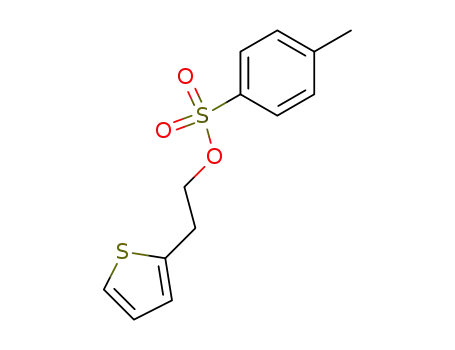
2-(2-thienyl)ethyl tosylate
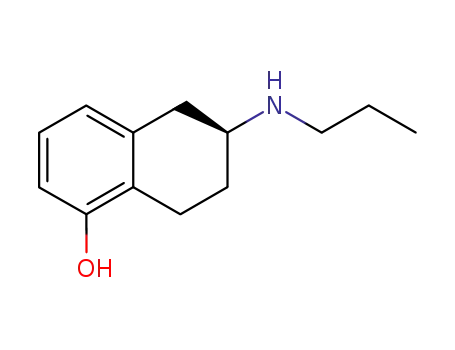
Desthienyl-Rotigotine
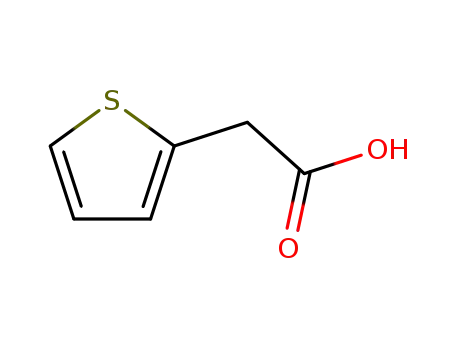
Thiophene-2-acetic acid

rotigotine hydrochloride
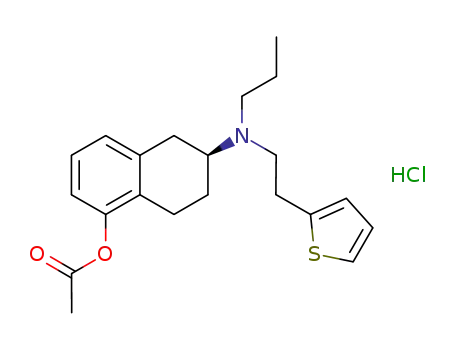
(S)-6-(propyl(2-(thiophen-2-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-yl acetate hydrochloride
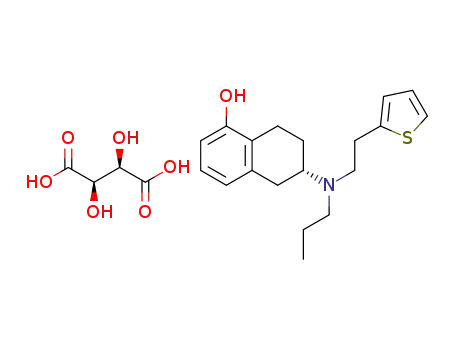
(S)-6-(propyl(2-thiophen-2-ylethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol hydrogen tartrate
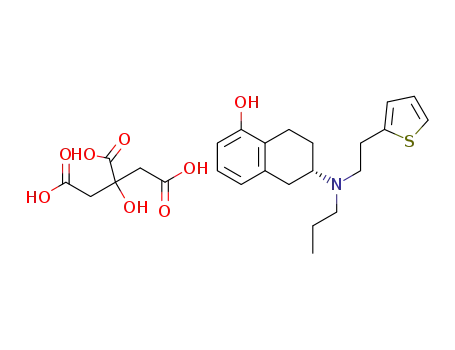
(S)-6-(propyl(2-thiophen-2-ylethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol dihydrogen citrate