Home > Products > Human APIs
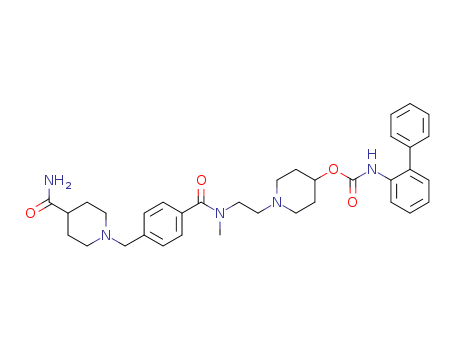

CasNo: 864750-70-9
MF: C35H43N5O4
The invention discloses a preparation me...
The invention relates to a synthesis met...
The invention provides a new rafenacin i...
The invention relates to the field of ph...
![[1,1'-biphenyl]-2-yl-isocyanate](/upload/2025/8/90e36342-b236-4883-9e35-5a9ef6702cfd.png)
[1,1'-biphenyl]-2-yl-isocyanate

![biphenyl-2-yl carbamic acid 1-(2-{[4-((4 carbamoylpiperidin-1-yl)methyl)benzoyl]N-methylamino}ethyl)piperidin-4-yl ester](/upload/2025/8/95000150-a836-4aed-a3fe-0e69af158def.png)
biphenyl-2-yl carbamic acid 1-(2-{[4-((4 carbamoylpiperidin-1-yl)methyl)benzoyl]N-methylamino}ethyl)piperidin-4-yl ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1.1: 12 h / 70 °C
1.2: 12 h / 20 - 40 °C / Inert atmosphere
1.3: pH 12
2.1: 2-methyltetrahydrofuran / 3 - 30 °C
2.2: 7 - 20 °C
2.3: 0.33 h / 20 °C
3.1: hydrogen / palladium 10% on activated carbon / 2-methyltetrahydrofuran; methanol / 3.02 h / 760.05 Torr / Sealed tube
4.1: 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride / 2-methyltetrahydrofuran / 20 °C
4.2: 0.17 h
5.1: isopropyl alcohol / 60 °C
5.2: 2.08 h / 18 - 25 °C
5.3: 0.25 h
With
hydrogen; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride;
palladium 10% on activated carbon;
In
2-methyltetrahydrofuran; methanol; isopropyl alcohol;
|
C17H23N3O3

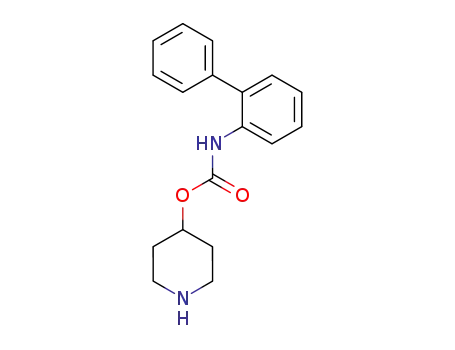
4-piperidyl N-(2-biphenyl)carbamate

![biphenyl-2-yl carbamic acid 1-(2-{[4-((4 carbamoylpiperidin-1-yl)methyl)benzoyl]N-methylamino}ethyl)piperidin-4-yl ester](/upload/2025/8/95000150-a836-4aed-a3fe-0e69af158def.png)
biphenyl-2-yl carbamic acid 1-(2-{[4-((4 carbamoylpiperidin-1-yl)methyl)benzoyl]N-methylamino}ethyl)piperidin-4-yl ester
| Conditions | Yield |
|---|---|
|
C17H23N3O3; 4-piperidyl N-(2-biphenyl)carbamate;
With
acetic acid; sodium sulfate;
In
tetrahydrofuran;
at 25 ℃;
for 2h;
With
sodium tris(acetoxy)borohydride;
In
tetrahydrofuran;
at 0 - 25 ℃;
for 2h;
Temperature;
|
85% |
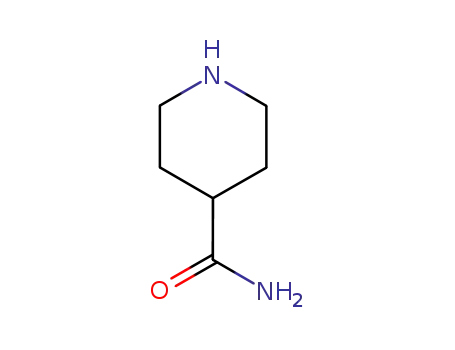
4-carboxamidopiperidine
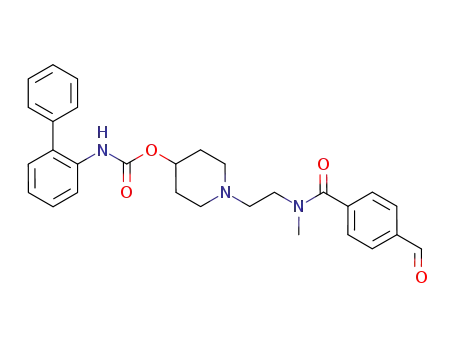
biphenyl-2-yl-carbamic acid 1-{2-[(4-formylbenzoyl)methylamino]ethyl}piperidin-4-yl ester
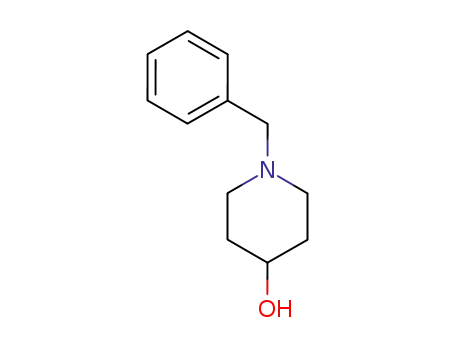
1-benzyl-4-hydroxypiperidine
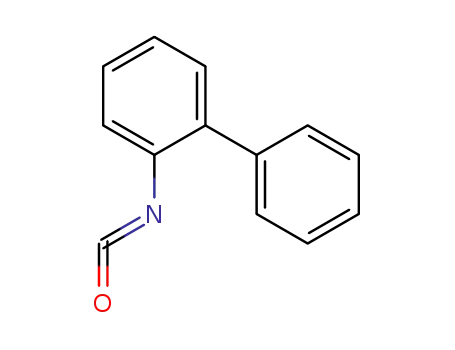
[1,1'-biphenyl]-2-yl-isocyanate