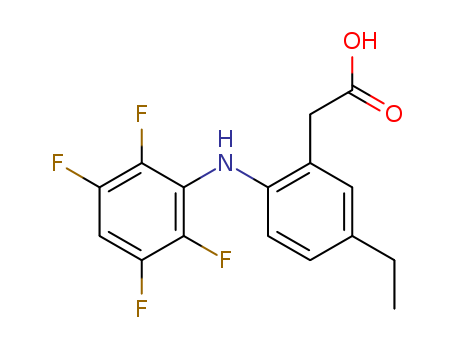

CasNo: 220991-32-2
MF: C16H13 F4 N O2
|
Definition |
ChEBI: An aromatic amino acid that is 2-amino-5-ethylphenylacetic acid in which one of the amino hydrogens is replaced by a 2,3,5,6-tetrafluorophenyl group. A selective cyclooxygenase 2 inhibitor that is used in veterinary medicine for the relief of pain and infl mmation in cats and dogs. |
InChI:InChI=1/C16H13F4NO2/c1-2-8-3-4-12(9(5-8)6-13(22)23)21-16-14(19)10(17)7-11(18)15(16)20/h3-5,7,21H,2,6H2,1H3,(H,22,23)
The invention relates to a preparation m...
The invention relates to a preparation m...
The invention belongs to the technical f...
The invention relates to a method for pr...
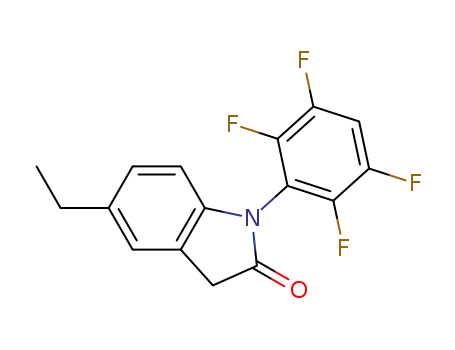
N-(2’3’5’6’-tetrafluorophenyl)-5-ethylindol-2-one

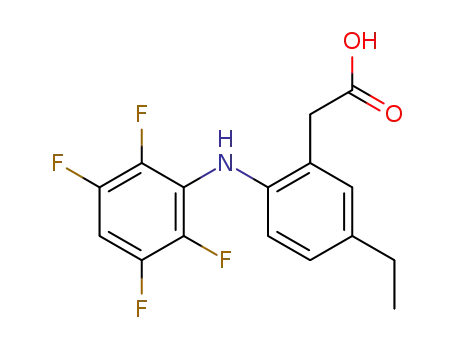
5-ethyl-2-(2',3',5',6'-tetrafluoroanilino)-phenylacetic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
ethanol; water;
at 80 ℃;
for 5h;
Temperature;
Reagent/catalyst;
Solvent;
|
98.7% |
|
With
potassium hydroxide;
In
methanol; water;
at 80 ℃;
for 5h;
Solvent;
Reagent/catalyst;
|
98.3% |
|
N-(2’3’5’6’-tetrafluorophenyl)-5-ethylindol-2-one;
With
water; sodium hydroxide;
In
toluene;
at 50 ℃;
for 0.333333h;
With
acetic acid;
In
water; isopropyl alcohol;
at 20 ℃;
for 0.5h;
pH=4 - 5;
|
81% |
|
With
sodium hydroxide;
In
methanol; water; toluene;
|
|
|
N-(2’3’5’6’-tetrafluorophenyl)-5-ethylindol-2-one;
With
sodium hydroxide;
With
hydrogenchloride;
|
C16H9F4NO2


5-ethyl-2-(2',3',5',6'-tetrafluoroanilino)-phenylacetic acid
| Conditions | Yield |
|---|---|
|
C16H9F4NO2;
With
sodium hydroxide;
In
water; toluene;
at 50 ℃;
for 0.333333h;
With
hydrazine hydrate;
In
water; toluene;
for 0.5h;
Reflux;
With
potassium hydroxide;
for 1.5h;
Reflux;
|
91% |

N-(2’3’5’6’-tetrafluorophenyl)-5-ethylindol-2-one
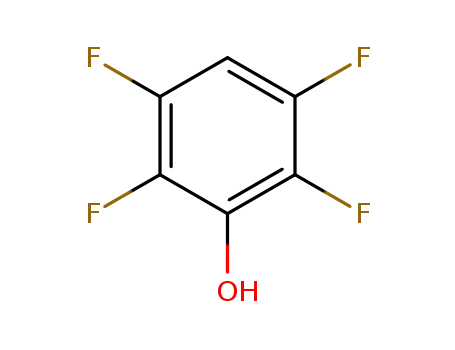
2,3,5,6-tetraflourophenol
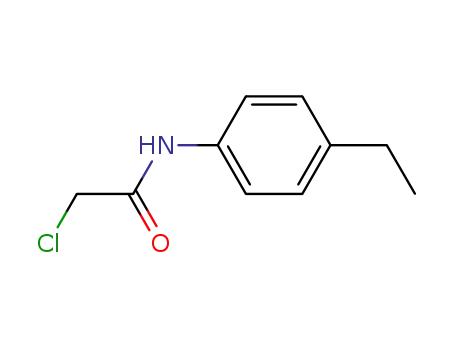
α-chloro-N-(p-ethylphenyl)acetamide
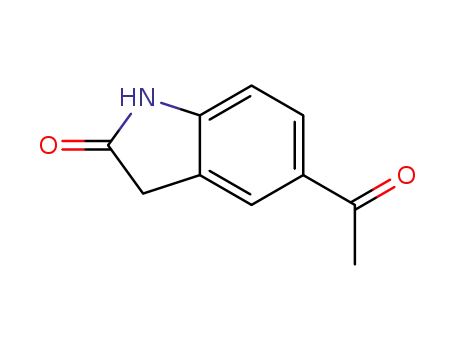
5-acetylindolin-2-one