Home > Products > Human APIs
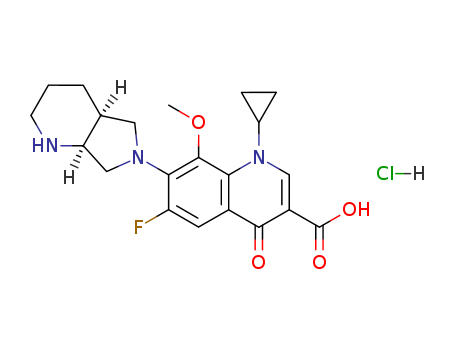

CasNo: 186826-86-8
MF: C21H24FN3O4.ClH
Appearance: 99%
Quality products make an important contribution to long-term revenue and profitability. Buy cost-effective 99% pure Moxifloxacin HCl 186826-86-8 now
An antibacterial agent that inhibits the activities of Topo II (DNA gyrase) and topoisomerase IV
(4aS-cis)-1-cyclopropyl-7-(2,8-diazabicyclo-[4.3.0]non-8-yl)-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinoline carboxylic acid-O3,O4(bis(acyloxy-O)) borate

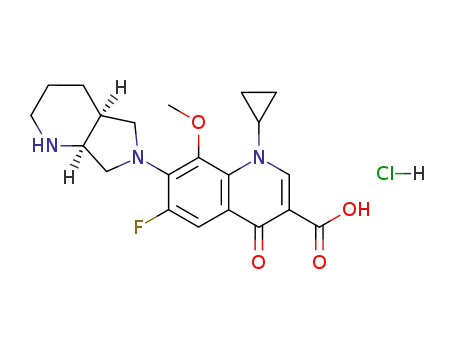
moxifloxacin hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol; water;
at 0 - 8 ℃;
for 0.333333h;
Temperature;
|
96.2% |
|
With
hydrogenchloride;
In
water;
at 0 ℃;
for 4h;
pH=< 1;
|
96.5% |
|
With
hydrogenchloride;
In
methanol;
at -5 - 20 ℃;
for 2h;
pH=1;
Temperature;
|
93.1% |
|
With
sodium hydroxide;
In
acetone;
for 1h;
Large scale;
|
89.8% |
|
With
sodium hydroxide;
at 80 ℃;
for 2.5h;
|
84.75% |
|
With
hydrogenchloride;
In
water;
at 10 - 15 ℃;
for 6h;
pH=1.0 - 2.0;
|
118 g |
|
With
hydrogenchloride;
In
ethanol; water;
at 25 ℃;
for 1h;
pH=1;
Temperature;
|
48 g |
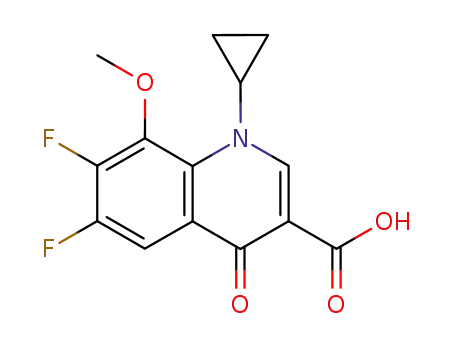
1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid

![(1S,6S)-2,8-diazabicyclo[4.3.0]nonane](/upload/2025/8/30aa804a-c799-47c4-a2ad-3eb4ef142f46.png)
(1S,6S)-2,8-diazabicyclo[4.3.0]nonane


moxifloxacin hydrochloride
| Conditions | Yield |
|---|---|
|
1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid; (1S,6S)-2,8-diazabicyclo[4.3.0]nonane;
With
titanium(IV) isopropylate; triethylamine;
In
isopropyl alcohol;
at 100 ℃;
Inert atmosphere;
With
hydrogenchloride;
In
methanol;
at 20 ℃;
for 1h;
pH=1;
Solvent;
Reagent/catalyst;
|
90% |
|
Multi-step reaction with 2 steps
1: Alkaline conditions
2: hydrogenchloride
With
hydrogenchloride;
|
|
|
1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid; (1S,6S)-2,8-diazabicyclo[4.3.0]nonane;
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
acetonitrile;
at 20 - 85 ℃;
for 36h;
Inert atmosphere;
With
hydrogenchloride;
In
water;
at 15 ℃;
for 1h;
pH=1.4 - 1.8;
Inert atmosphere;
|
3.3 g |
The CAS number of Moxifloxacin hydrochloride is 186826-86-8.
More information of Moxifloxacin hydrochloride 186826-86-8 are:
|
CAS Number |
186826-86-8 |
|
Melting Point |
Slightly yellow to yellow crystalline powder, mp 324-325° |
|
Boiling Point |
636.4 °C at 760 mmHg |
|
Flash Point |
338.7 °C |
|
Vapor Pressure |
4.56E-17mmHg at 25°C |
|
HS CODE |
2933499090 |
|
PSA |
83.80000 |
|
LogP |
3.56630 |
Synonyms for Moxifloxacin hydrochloride 186826-86-8:Moxifloxacin HCl;3-Quinolinecarboxylicacid,1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-,hydrochloride (1:1);3-Quinolinecarboxylic acid,1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-,monohydrochloride (9CI);Actira;Avalox;Avelox;BAY 12-8039;Lapinix;Octegra;
The chemical formula of Moxifloxacin hydrochloride is C21H24FN3O4.ClH which containing 21 Carbon atoms,25 Hydrogen atoms,1 Fluorine atoms,3 Nitrogen atoms,1 Oxygen atoms and 1 Chlorine atoms,and the molecular weight of Moxifloxacin hydrochloride is 437.899.
Moxifioxacin hydrochlodde is one of the two new fluoroquinolonecarboxylic acid antibiotics introduced in 1999 for the treatment of respiratory tract infections such as community-acquired pneumonia, acute exacerbations of bronchitis or acute sinusitis. Moxifloxacin can be synthesized through a 12 step sequence from the classical 4-quinolone-3-carboxylic acid template. Some advantages of Moxifloxacin over Ciprofloxacin, another of Bayer's launched quinolones, have been shown, i.e. an enhanced activity against Gram-positive bacteria (Streptococcus pneumoniae, Clostndium pneumoniae), a favorable pharmacokinetic profile (good tissue penetration and plasma concentrations above MICs) and a lack of phototoxicity (UVA irradiation).
InChI:InChI=1/C21H24FN3O4.ClH/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24;/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28);1H
Relevant articles related to Moxifloxacin hydrochloride:
|
Article |
Source |
|
Synthetic method of moxifloxacin hydrochloride |
- Paragraph 0037-0057, (2020/07/13) |
|
Preparation method of moxifloxacin hydrochloride (by machine translation) |
- Paragraph 0048-0055; 0066-0080, (2020/05/01) |
HANGZHOU CLAKE BIOTECH CO.,LTD is a quality supplier of Moxifloxacin hydrochloride. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on Moxifloxacin hydrochloride 186826-86-8.