Home > Products > Human APIs
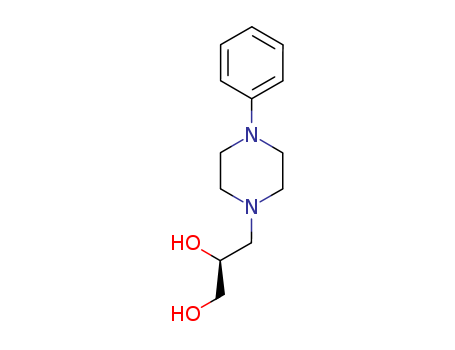

CasNo: 99291-25-5
MF: C13H20N2O2
Appearance: white solid
|
Definition |
ChEBI: A member of the class of N-arylpiperazines that is N-phenylpiperazine in which the amino hydrogen is replaced by a 2,3-dihydroxypropyl group (the S-enantiomer). A peripherally acting antitussive drug t at is used as an alternative to opioids. |
InChI:InChI=1/C13H20N2O2/c16-11-13(17)10-14-6-8-15(9-7-14)12-4-2-1-3-5-12/h1-5,13,16-17H,6-11H2/t13-/m0/s1
A series of hydroxy-functionalized phosp...
The invention belongs to the technical f...
Levodropropizine, an antitussive drug, w...
A process for the optical resolution of ...
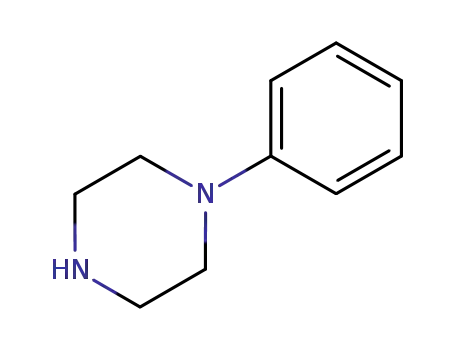
4-phenyl-1-piperazine

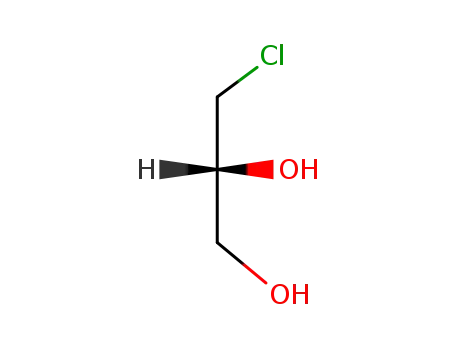
(2R)-3-chloro-1,2-propanediol

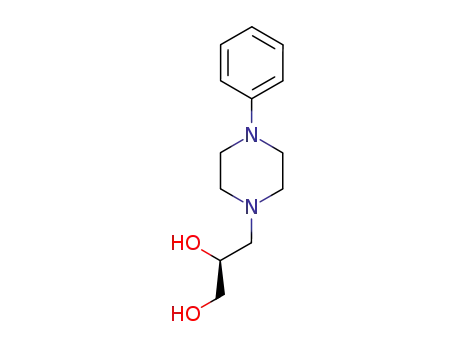
levodropropizine
| Conditions | Yield |
|---|---|
|
In
water;
at 10 - 42 ℃;
for 12.5h;
Temperature;
Cooling with ice;
Large scale;
|
80.6% |

4-phenyl-1-piperazine


levodropropizine
| Conditions | Yield |
|---|---|
|
In
benzene;
|
|
|
In
dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: sodium hydroxide / water / 0.25 h / 75 °C
2: (2-hydroxyphenyl)diphenyl(propyl)phosphonium iodide / neat (no solvent) / 24 h / 45 °C / 7500.75 Torr / Autoclave
3: sodium hydroxide / water / 3 h / 23 °C
With
(2-hydroxyphenyl)diphenyl(propyl)phosphonium iodide; sodium hydroxide;
In
water;
|
|
|
Multi-step reaction with 3 steps
1: sodium hydroxide / water / 0.25 h / 75 °C
2: (2-hydroxyphenyl)diphenyl(propyl)phosphonium iodide / neat (no solvent) / 48 h / 23 °C / 7500.75 Torr / Autoclave
3: sodium hydroxide / water / 3 h / 23 °C
With
(2-hydroxyphenyl)diphenyl(propyl)phosphonium iodide; sodium hydroxide;
In
water;
|

4-phenyl-1-piperazine
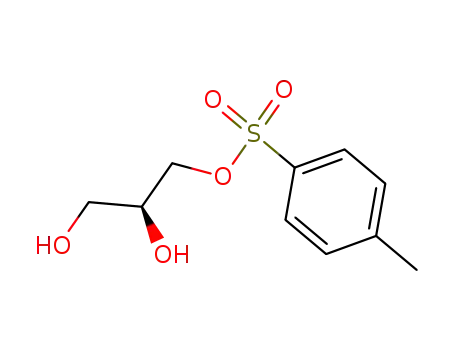
(S)-1-tosyloxy-2,3-propanediol
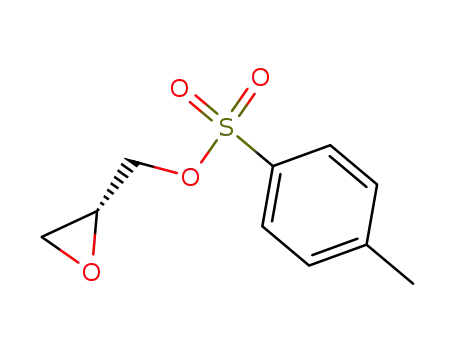
(R)-glycidyl tosylate

(2R)-3-chloro-1,2-propanediol